Clonal Hematopoiesis of indeterminate potential (CHIP) is a condition where blood cells are produced from a few clones of hematopoietic stem cells (HSCs) carrying leukemia-associated driver mutations 1. Although selective pressures from low-grade inflammation of the bone marrow microenvironment have been shown to promote mutant cell expansion 2-7, we focus on the disease-initiating niche, which is defined as a subset of marrow cells surrounding rare HSCs. Indeed, as we have recently described, clonal expansion of acute myeloid leukemia and activated HSCs 8,9 was not uniform, and only occurred in a subset of marrow spaces harboring bone remodeling activities. To identify the disease-initiating niche for therapeutic targeting, we developed an intravital imaging protocol to visualize the rare pre-malignant clones in the minimally perturbed microenvironment and phenotype their niche.
The standard practice to generate CHIP models relies on transplanting syngeneic or allogeneic cells into a sub-lethally irradiated recipient. However, concerns arise as irradiation compromises the microenvironment 10. Successful engraftment in non-conditioned mice can be achieved by injecting a high number of cells (1.5 x 10 7 cells) 11,12, but modeling early clonal expansion events from single cells remain challenging, as a crowd of cells is often observed in proximity. Here, we established a low dose (0.5-1.5 Gy), whole body or local irradiation regimen that preserves the integrity of the bone marrow microenvironment. We performed high-resolution in-vivo imaging to capture dynamics of single cells and cells undergoing early expansion. Specifically, as little as 0.5Gy irradiation enabled survival of the transplanted cells (2x10 6 GFP + healthy whole bone marrow) in non-irradiated side, and allowed direct visualization of early clonal expansion in vivo through 16 weeks . Engraftment was negligible in non-irradiated mice.Through in-vivo, video-rate tracking of Rhodamine dextran dye (70kDa) leakage from vessels into the extravascular space showed no increase in permeability 13 or signs of vessel dilation, suggesting minimal inflammation after the radiation insult (N= 3 mice, data points are individual vessel segments. Mann-Whitney U test). Preliminary findings with in-vitro cultures of mesenchymal stromal cells (MSCs) have also suggested that the MSCs retain their ability for tri-lineage differentiation after the low-dose irradiation. With this imaging protocol, we showed that hot spots of cell expansion exist in the Tet2 +/- murine model.
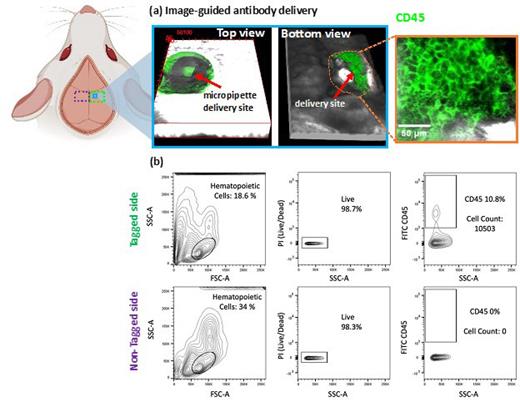
To capture the highly localized niche factors responsible for these hot spots, we implemented image-guided live-cell labeling adapted from the previously published Image-seq protocols 9. As illustrated in Figure 1, a small channel is etched in the bone with plasma-mediated laser ablation. The micropipette was then inserted to the region of interest for delivery of fluorescent antibodies under image guidance. By administering FITC-conjugated anti-CD45 antibodies into the marrow cavities, we can label and recover ~10,000 live cells with 98% viability from two regions of interest, suitable for next generation single cell sequencing. The technique can be expanded to employ antibody cocktails to visualize and enrich particular cell lineages of interest during cell isolation, while preserving spatial information. Its compatibility with standard cell isolation protocols for FACS may help collect cellular targets that are geometrically inaccessible for cell aspiration.
In conclusion, we established a working model to visualize expansion of healthy and Tet2 +/- hematopoietic cells in vivo. This can be followed by image-assisted live-cell tagging to isolate local microenvironment cells and study cell-niche coordination at high spatial precision. The imaging protocol may also be broadly applied to study microenvironment regulations in non-malignant clonal disorders.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal